Hospital germs
Resistant bacteria are particularly problematic in hospitals, as they can infect vulnerable people who are susceptible to infections. The fact is that many sick people living closely together can lead to serious outbreaks. Who are the experts to approach when trying to assess the hygiene of a hospital? Which information is important and which studies are relevant? We provide an overview.
On this page: Data and organisations | Experts | Important studies
Data and organisations
World Health Organisation
The WHO is run by the United Nations and focuses on global healthcare. It published a global overview of antibiotic resistance. It also publishes recommendations on the use of antibiotics and hygiene.
European Centre for Disease Prevention and Control
Central authority dealing with infectious diseases. Publishes an annual report with data on resistant germs in European hospitals.
Experts

Germany
Mathias Bonk, Global Health
Think Global Health
Dr. med. Mathias Bernhard Bonk
Dangersen Dorf 24
21244 Buchholz
Matthias Bonk is an independent global health consultant. He is currently working at the WHO regional office in Berlin and at Charite Berlin. Together with the Lancet, a scientific journal, he organises the New Voices in Global Health programme. He has also worked as a paediatrician and neonatologist in Germany, the United Kingdom and India. As an independent global health consultant his aim is to provide strong and up-to-date evidence of threats to global health for decision makers, the public and specialists. He is also concerned about the problem of antibiotic resistance.
Tim Eckmanns, Hospital Germs
Robert Koch-Institut
Nordufer 20
13353 Berlin
Phone: +4930 / 18754-3485
Tim Eckmanns is head of the Hospital Infections, Surveillance of Antibiotic Resistance and Consumption department at the Robert Koch Institute in Berlin. The sector focuses on the surveillance of antibiotic resistance and consumption as well as nosocomial outbreaks. Nosocomial outbreaks are outbreaks of hospital infections. At the time of these outbreaks Eckmanns supports the authorities responsible with the outbreaks‘ management and evaluation.
Petra Gastmeier, Hospital Hygiene
Institut für Hygiene und Umweltmedizin
der Charité - Universitätsmedizin Berlin
Humboldt Universität Berlin
Campus Benjamin Franklin
Hindenburgdamm 27
12203 Berlin
Phone: +49 30 450 577 612
Petra Gastmeier is head of the institute for hygiene and environmental medicine at Berlin’s Charité. She is also an expert in hospital hygiene. In 2015 she received the Robert Koch Prize for hospital hygiene and prevention of infections. The RKI said: Professor Gastmeier has fought infections in hospitals and has worked towards better hygiene with special commitment. She established the hospital surveillance system KISS in order to make hospitals take hygiene more seriously. She continues her fight against bad hygiene in hospitals with her campaign “Action on Clean Hands.” Gastmeier is also head of the reference centre for the surveillance of hospital infections.
Christoph Lübbert, Hospital Germs
Universitätsklinikum Leipzig AöR
Department für Innere Medizin, Neurologie und Dermatologie
Klinik für Gastroenterologie und Rheumatologie
Fachbereich Infektions- und Tropenmedizin
Liebigstraße 20
04103 Leipzig
Phone: 0049 341 / 97-24970 or 12222
Christoph Lübbert is head of the Infection and Tropical Medicine department at the University Clinic of Leipzig. He has a degree in tropical medicine and public health from the Liverpool School of Tropical Medicine in the UK. As an infectious diseases specialist he also deals with multi resistant germs. In 2016 he published a study about carbapenem-resistant Enterobacteria which are brought to Northern Europe from southern European countries like Greece and Italy.
Sweden
Otto Cars, Hospital Germs
Department of Medical Science, Infectious Diseases and Antiobiotics Research
Uppsala University
Akademiska sjukhuset
751 85 Uppsala
Phone: +46 70 8920203
Otto Cars is professor at the Department of Medical Science, Infectious Diseases and Antiobiotics Research at Uppsala University. For more than twenty years he has been drawing attention to the issue of antibiotic resistance. Cars is founder of the international network ReAct in Uppsala, which has recently launched a toolbox for responsible use of antibiotics. In 2015, he was awarded the Swedish King’s Medal for his persistent commitment against the threat of antibiotic resistance.
USA
John H. Rex, Presidential Advisory Council on Combating Antibiotic-Resistant Bacteria
Office of the Assistant Secretary for Health
Office of Communications
Department of Health and Human Services
Phone: +1 (202) 205-0143
John Rex is a member of the Presidential Advisory Council on Combatting Antibiotic Resistant Bacteria, and Chief Strategy Officer at the pharmaceutical company AstraZeneca. He has also worked for several smaller pharmaceutical companies. With his experience he can bring together representatives of the pharmaceutical industry and scientists. For example, he is one of two co-founders of the New Drugs for Bad Bugs programme, where scientists and pharmaceutical companies collaborate in order to find and develop new antibiotics.
Studies
Will 10 Million People Die a Year due to Antimicrobial Resistance by 2050?
A high profile report on antimicrobial resistance from the UK Review on Antimicrobial Resistance – commissioned by David Cameron and led by famous economist Lord Jim O’Neill – estimated that 10 million people would die every year by 2050 from antimicrobial resistance.
The 10 million deaths figure is quoted by global leaders, the media, campaigners and experts. Often the caveats and uncertainties attached to this ‘specific, frightening conclusion’ are omitted. The authors of an article published in Plos Medicine argue that this unreliable global deaths estimate undermines the fight against a post-antibiotic era, rather than supporting it.
The article acknowledges the massive public health burden of the issue, but the lays out the uncertainties associated with the 10 million figure. The authors argue that detailed and reliable global data is needed both to combat the problem and to come up with more accurate predictions of it’s future impact.
Every year more than two million European citizens get an infection while they’re in hospital
Prevalence of hospital infections in Europe. Learn more
A wide-ranging study led by Alessandro Cassini from the European Centre for Disease Prevention and Control (ECDC) found that 90,000 people die each year from infections they pick up while they’re in hospital.
This was the first time that scientists have calculated the number of lives lost due to hospital infections. They also found that hospital infections have a bigger impact on the health system than tuberculosis, flu and HIV. Hospital infections were the worst of all the infections the ECDC monitors.
Hospital infections are mostly due to lack of hygiene – poor hand washing and surgical instrument cleaning by doctors. This means that hospital infections are mostly avoidable.
Doctors are being told to prescribe fewer antibiotics
Most antibiotics aren’t taken in hospital, they’re taken in peoples homes. In the UK the difference is roughly 80 per cent at home to 20 per cent in hospital. But a lot of the antibiotics taken at home are unnecessary.
How can we end this problem? According to researchers at the Behavioural Insights Team in London, led by Michael Hallsworth, it’s surprisingly easy. The researchers first worked out which doctors surgeries were prescribing the most antibiotics in their area. They got this data from public databases.
Then they separated this group of almost 1,600 doctors surgeries into two groups. The scientists wrote a letter to every second medical practice explaining to the doctors that they were prescribing a particularly high number of antibiotics. The researchers said that this simple psychological trick made the affected doctors (the ones written to) feel like outsiders. They also added a note from the Chief Medical Officer to the letter.
This simple measure has already had an affect: the prescription of antibiotics in the medical practices written to by the researchers decreased by three per cent in comparison to the group of doctors who did not receive a letter.
Three percent might not sound like much. However, three per cent of almost 800 practices amounts to more than 70,000 fewer prescriptions of antibiotics in six months, according to expert estimates. This would save the NHS almost £100,000 pounds, at an expense of less than 5,000 Euros for printing and sending the brochures.
However the study also shows another obstacle in the way of reducing antibiotic consumption: it is much harder to explain the problem of needlessly swallowed antibiotics to patients. In a second experiment, the researchers again separated the 1,600 practices into two groups. One half received posters and brochures to be used to inform patients about the issue. This time, there was no change and the rate of antibiotic prescriptions remained high.
The researchers concluded that either the patients targeted by the study were unwilling to listen, or they’d already been given so much information on antibiotic use that the brochure didn’t make a difference. When one of the main global efforts to reduce antibiotic consumption is targeted at patients, this is far from a good message.
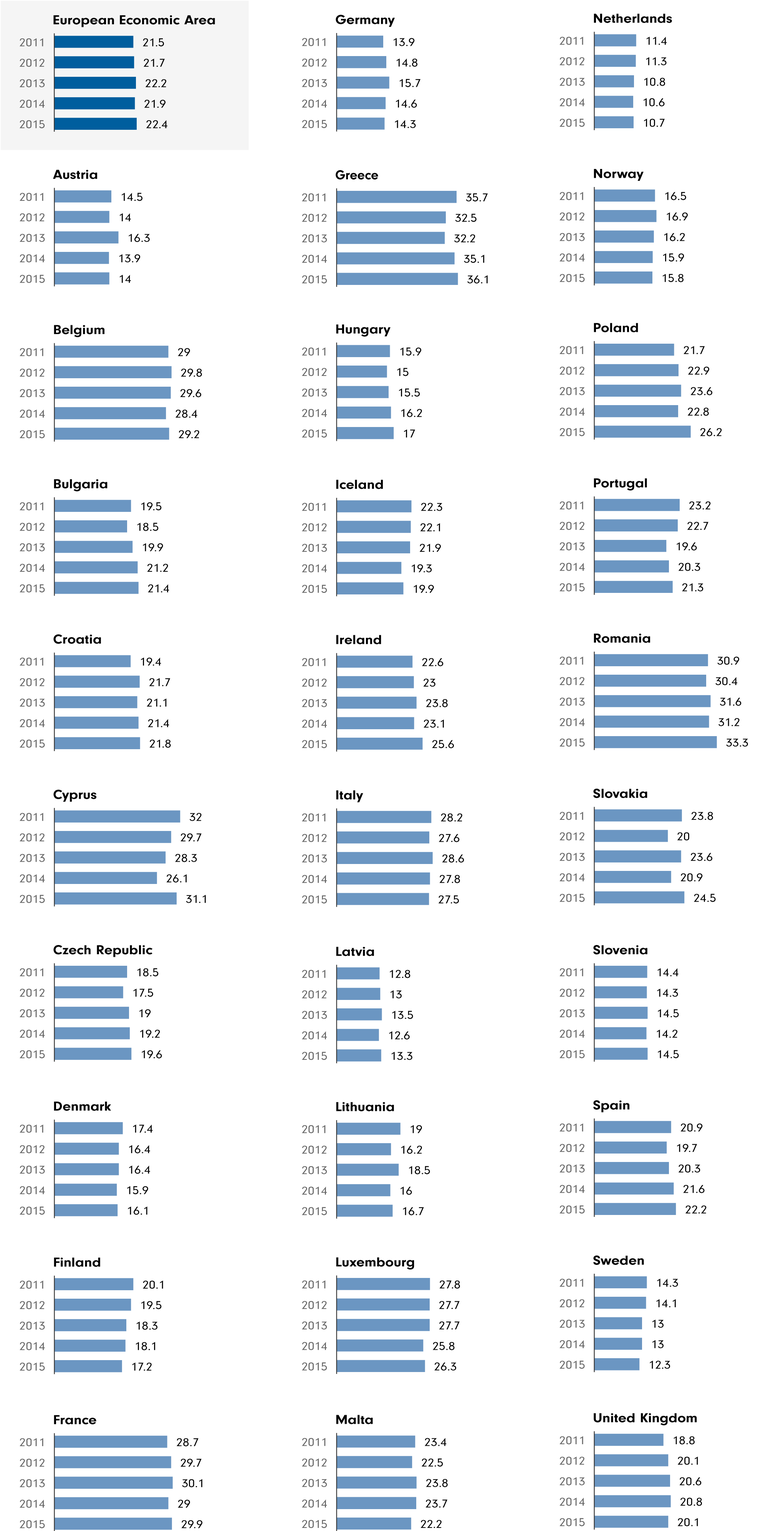
Antibiotic consumption in Europe. Learn more
Doctors, in contrast, seem to be more receptive to advice. The British researchers expect the awareness campaign to reduce antibiotic consumption outside hospitals by almost one per cent in the short term. The government is trying to reduced antibiotic consumption by four per cent, so this would be a big step in the right direction.
Whether this campaign would work in other countries is uncertain. The UK has a big advantage because prescription data for each doctors practice is publicly available. In Germany, for example, this is not the case.
Resistant bugs can be picked up travelling – but in most cases they disappear rapidly
Travellers to exotic destinations often unknowingly bring resistant germs home with them in their gut. Scientists from the Etienne Ruppé of the Diderot University in Paris researched the phenomenon. They analysed the stool of over 800 travellers and published their results in August 2015. The results: one out of two tourists were carrying resistant germs. For travellers who visited Asia the rate was as high as 70 per cent. The good news was that the germs did not last long in the guts of the returning travellers – even without antibiotic treatment. After three months, 95 per cent were free of resistant germs.
Scientists may soon be able to identify germs faster
There is often a difference between what is good for an individual patient and what is good for reducing antibiotic resistance. Doctors generally want to administer a patient with an antibiotic that kills as many different germs as possible, as quickly as possible. When treatment begins it is often unclear which bacteria is causing the infection, and normal identification methods can take days. This is why doctors choose to save their patients by using the strongest possible antibiotic on the infection. This practice, however, increases the risk in the long run. This method increases the likelihood of germs developing resistance to an antibiotic. Ideally, doctors could choose their weapon of choice with precision.
That has now become more likely. Scientists led by Ritu Banerjee from the Mayo Clinic in Rochester, Minnesota, have tested a method for rapid identification of germs. Within two hours, they could identify the bugs and any possible resistance.
Combining two antibiotics can be effective against resistant germs
Helicobacter pylori is a dangerous bug. It infects the stomach and gut, causes ulcers and even cancer. And like many bacteria, it develops resistance to the antibiotics used to treat it. Scientists led by Jyh-Chin Yang of the National Taiwan University in Taipei have now successfully treated the germ by using a combination of two substances. Not only does this method make the treatment more efficient – it also helps circumvent the development of resistance.
It has been known that combination therapy with two or more antibiotics can lead to less resistance; it is more difficult for germs to adapt to different substances at the same time. However, combination therapy is unpopular. These therapies are more complicated for doctors to use than a single drug.
Sometimes resistance can develop very rapidly – and then disappear as rapidly as it developed
Scientists led by Jakob Haaber of Stanford University have discovered a temporary resistance mechanism. Normally, resistance arises when genes mutate. These changes are long lasting, hereditary and are discovered though genetic analysis in the lab.
Haaber’s team discovered something else. They found that when Staphylococcus aureus, a germ commonly seen in wounds, is treated with colistin, it also develops resistance to the antibiotic vancomycin. Yet this resistance does not last. It disappears when colistin is withdrawn. The scientists concluded that this mechanism can interfere with antibiotic treatment – under the radar of conventional clinical analysis.
Urinary tract pathogens are widely spread
Little is known about resistance outside of hospitals. That is why researchers led by Dean Ironmonger of the Field Epidemiology Service in Birmingham analyzed more than five million urinary tract infection samples. Over five years, they discovered a rise in resistance to the antibiotic cephalosporin. Escherichia coli and Klebsiella pneumoniae were two of the germs affected. This is the first systematic analysis of antibiotic resistance in the English community. And it is troubling.
Disinfectants can lead to resistances

Disinfectant use can also lead to antibiotic resistance, scientists led by Mark Webber of the University of Birmingham discovered. They treated the typhus germ Salmonella typhimurium with different disinfectants. The result was the emergence of bacterial strains immune to the disinfectants. These strains were also resistant to antibiotics.
The reason for the double resistance: The bacteria had acquired molecular pumps that expel the disinfectants and antibiotics from the bacterial cell. The results should encourage frugal use of disinfectants, especially at home, where there is hardly any evidence for their effectiveness.
Mold can become resistant
Scientists led by Oliver Bader of the University of Göttingen in Germany have discovered resistance in a fungus taken from soil samples. The fungus, Aspergillus fumigatus, can infect immunodeficient patients. Normally treated with azole, resistance to the substance has spread in recent years. It is unknown how common resistance to the anti fungal is, but the scientists provided an estimate in their study: One in every ten fungi they isolated from the soil was resistant.
A new antibiotic called griselimycin
Good news: Scientists led by Angela Kling of the Helmholtz Institute for Pharmaceutical Research in Saarbrücken, Germany, have developed a synthetic antibiotic called griselimycin. The substance could possibly treat tuberculosis. It has been effective in lab experiments involving mice, but has yet to be developed for treating humans.
The background: Normally, the antibiotic streptomycin is used to fight Mycobacterium tuberculosis, the germ which causes the disease. But certain strains have developed resistance. Streptomycin was first discovered in a fungus. This fungus, Streptomyces, also naturally produces griselomycin. However, natural griselimycin is not suitable as a drug because it decomposes too quickly. But with the new synthetic modifications, it may have a future career in medicine.
New resistance against colistin
Colistin is an antibiotic that can cause strong side effects in humans. This is why it is only used as a last resort, when other antibiotics have been ineffective. Because it is used so infrequently, resistance has hardly developed.
But colistin has been used widely in farming. This wasn’t thought of as a problem, until scientists discovered that resistance had developed. At first, because the resistance gene was found on a non-mobile part of the bacterial DNA, it was thought that the resistance could not be transferred from one bacteria to another. But Chinese scientists became suspicious after observing the spread of resistant germs in pig farms. Could resistance be transferred from germ to germ after all? Yes. The scientists collected samples and discovered a resistance gene located on a mobile part of the DNA. They called it mcr-1.
Since then the resistant gene has been found in other countries, as well as in humans. The discovery has spurred debate over whether the use of colistin in farming should be reduced.
New approaches for the production of antibiotics
Many antibiotics have been isolated from bacteria that live in the soil. These bacteria have learned through evolution to use antibiotics to poison other microbes. The substances normally don’t harm humans, that’s why they can be used as medicine.
Over time, this source – the soil that provided antibiotics – seemed to run dry. The question was, have we already discovered all the organisms that can act as producers of antibiotics? No, say scientists led by Losee Ling from NovoBiotic Pharmaceuticals in Cambridge, Massachusetts. Normally, newly discovered bacteria are grown on petri dishes in the lab, but what if a large portion of microorganisms do not grow there?
Based on this idea, the scientists collected samples of bacteria from the soil, put them in containers and then placed them back into the earth. This let the microbes grow in their natural environment. In one of the containers, scientists identified a new substance: teixobactin, an antibiotic to which there is no known resistance yet. It is quite possibly the first of a whole array of substances to come from organisms that cannot be cultured in the lab.
